Chapters
Lodish 4th edition: Chapter 21 pages 935-944
Moyes and Schulte: Chapter 5 pages 165-168; 184-194
Nervous system overview - how neurons connect
Figure 21-6, Lodish 4th edition. A highly schematic diagram of the vertebrate nervous system. The central nervous system (CNS) comprises the brain and spinal cord. It receives direct sensory input from the eyes, nose, tongue, and ears. The peripheral nervous system (PNS) comprises three sets of neurons: (1) somatic and visceral sensory neurons, which relay information to the CNS from receptors in somatic and internal organs; (2) somatic motor neurons, which innervate voluntary skeletal muscles; and (3) autonomic motor neurons, which innervate the heart, the smooth involuntary muscles such as those surrounding the stomach and intestine, and glands such as the liver and pancreas. The sympathetic and parasympathetic autonomic motor neurons frequently cause opposite effects on internal organs. The cell bodies of somatic motor neurons are within the CNS; those of somatic sensory neurons and of autonomic motor neurons are in ganglia adjacent to the CNS.
Synaptic communication
Sherrington (1890) studied reflex functions of spinal cord
- coined the word synapse = a functional connection between surfaces
- synapse (from the Greek: to clasp, to connect or join)
- sites of interaction between neurons and neurons and their targets
Synapses can occur between a wide range of different regions of the neuron.


From Moyes and Schulte, Figure 5.27 Variation of the structure and location of synapses.
Electrical synapses
- cells connect via gap junctions:
- membranes are separated by 2 nm
- gap junctions link the cytosol of two cells and provide a passageway for
movement of very small molecules and ions between the cells - this can be
measured with a fluorescent dye and using a fluorescence microscope to observe
whether they pass into neighboring cells
Figure 21-35, Lodish 4th editon. An electric synapse. (a) The plasma membranes of the presynaptic and postsynaptic cells are linked by gap junctions. Flow of ions through these channels allows electric impulses to be transmitted directly from one cell to the other. (b) Negatively stained, electron microscopic image of the cytosolic face of a region of plasma membrane enriched in gap junctions; each “doughnut” forms a channel connecting two cells.
Figure 22-7, Lodish 4th edition. Electron micrograph of a thin section through a gap junction connecting two mouse liver cells. The two plasma membranes are closely associated for a distance of several hundred nanometers, separated by a “gap” of 2 3 nm.
- gap junction channels have a large conductance
- NO synaptic delay (current spread from cell to cell is instantaneous)
- important in some reflexes
- chemical synpases (below) do have a significant delay.
Figure 21-36, Lodish 4th edition. Transmission of action potentials across electric and chemical synapses. In both cases, the presynaptic neuron was stimulated and the membrane potential was measured in both the presynaptic and postsynaptic cells (see Figure 21-7a). Signals are transmitted across an electric synapse within a few microseconds because ions flow directly from the presynaptic cell to the postsynaptic cell through gap junctions. In contrast, signal transmission across a chemical synapse is delayed about 0.5 ms — the time required for secretion and diffusion of neurotransmitter and the response of the postsynaptic cell to it.
- commonly found in other cell types as well i.e. glia
- can be modulated by intracellular Ca+2, pH, membrane voltage, calmodulin
- clusters of proteins that span the gap such that ions and small molecules
can pass directly from one cell to another
-made up of 6 protein subunits arranged around a central pore, made up of
the connexin protein
- the two sides come together to make a complete unit of 12 proteins around
the central pore
- cloned from many tissues and organisms and are extremely well conserved
Figure 22-8, Lodish 4th edition. Structure of gap junctions. (a) In this model, a gap junction is a cluster of channels between two plasma membranes that are separated by a gap of about 2 3 nm. (b) Both membranes contain connexon hemichannels, cylinders of six dumbbell-shaped connexin subunits. (c) Each connexin subunit has four transmembrane alpha helices. Two connexons join in the gap between the cells to form a gap-junction channel, 1.5 2.0 nm in diameter, that connects the cytoplasm of the two cells.
Chemical
Figure 21-4, Lodish 4th edition. A chemical synapse.
(a) A narrow region — the synaptic cleft — separates the plasma membranes of the presynaptic and postsynaptic cells. Transmission of electric impulses requires release of a neurotransmitter (red circles) by the presynaptic cell, its diffusion across the synaptic cleft, and its binding by specific receptors on the plasma membrane of the postsynaptic cell. (b) Electron micrograph showing a cross section of a dendrite synapsing with an axon terminal filled with synaptic vesicles. In the synaptic region, the plasma membrane of the presynaptic cell is specialized for vesicle exocytosis; synaptic vesicles, which contain a neurotransmitter, are clustered in these regions. The opposing membrane of the postsynaptic cell (in this case, a neuron) contains receptors for the neurotransmitter.
Figure 21-30, Lodish 4th edition. Micrograph of an axon terminal obtained by the rapid-freezing deep-etch technique. Note the fibers, largely composed of synapsin, that interconnect the vesicles and also connect some to the active zone of the plasma membrane. Docked vesicles are ready to be exocytosed. Those toward the center of the terminal are in the process of being filled with neurotransmitter.
- most common type of synapse
- electrical signal in the presynaptic cell is communicated to the
postsynaptic cell by a chemical (the neurotransmitter),
- separation between presynaptic and postsynaptic membranes is about 20 to 30
nm
- a chemical transmitter is released and diffuses to bind to receptors on
postsynaptic side
- bind leads (directly or indirectly) to changes in the postsynaptic membrane
potential (usually by opening or closing transmitter sensitive ion channels)
- the response of the neurotransmitter receptor can depolarizes (excitatory
postsynaptic potential; epsp) or hyperpolarizes (inhibitory postsynaptic
potential; ipsp) the post-synaptic cell and changes its activity
- significant delay in signal (1 msec) but far more flexible than electrical
synapse
Some types of chemical synapse include:
i) Excitatory - excite (depolarize the postsynaptic cell)
ii) Inhibitory - inhibit (hyperpolarize the postsynaptic cell)
iv) Modulatory - modulates the postsynaptic cells response to other synapses
Figure 21-32, Lodish 4th edition. Excitatory and inhibitory responses in postsynaptic cells stimulated by acetylcholine. (a) Application of acetylcholine (or nicotine) to frog skeletal muscle produces a rapid postsynaptic depolarization of about 10 mV, which lasts 20 ms. The nicotinic acetylcholine receptors in these cells are ligand-gated cation channels; binding of acetylcholine opens the channel, admitting both Na+ and K+. (b) Application of acetylcholine (or muscarine) to frog heart muscle produces, after a lag period of about 40 ms (not visible in graph), a hyperpolarization of 2 3 mV, which lasts several seconds. These cells contain muscarinic acetylcholine receptors, which are coupled via a G protein to K+ channels. Activation of the receptor leads to channel opening.
| Different types of neurotransmitter receptors | ||
| Functional Type | Ligand* | Ion Channel |
| Excitatory Receptors | Acetylcholine (nicotinic receptor) | Na+/K+ |
| Glutamate (NMDA class receptors)† | Na+/K+ and Ca2+ | |
| Glutamate (non-NMDA class receptors)‡ | Na+/K+ | |
| Serotonin (5HT3 class receptors) | Na+/K+ | |
| Inhibitory Receptors | ?-Aminobutyric acid, GABA (A-class receptors) | Cl- |
| Glycine | Cl- | |
* Most of these ligands also bind to receptors
that are coupled to G proteins (see
Table 21-2). Their receptors that are ion channels are indicated in
parentheses. |
||
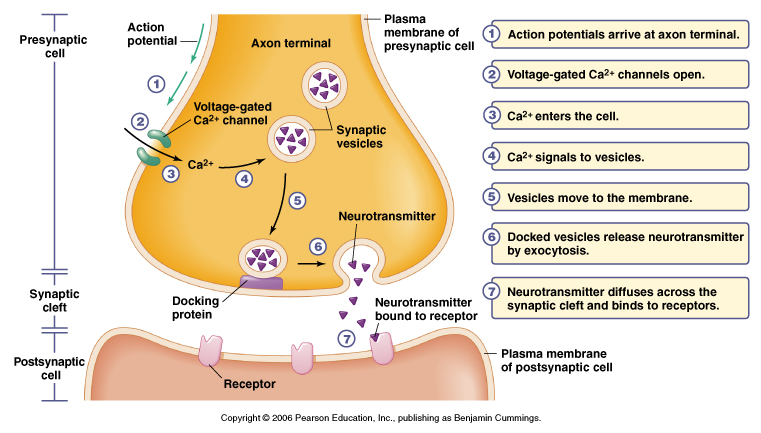
Figure 5.16 Events of signal transmission at a chemical synapse
From Moyes and Schulte
Basic Function of Chemical Synapse
1. Nerve impulse arrives at presynaptic terminal
2. Depolarization causes voltage-gated Ca+2 channels to open
- increases Ca+2 influx, get a transient elevation of internal Ca+2 ~100 mM
3. Vesicle exocytosis
- increase in Ca+2 induces fusion of synaptic vesicles to membrane
- vesicles contain neurotransmitters
4. Vesicle fusion to membrane releases stored neurotransmitter
5. Transmitter diffuses across cleft to postsynaptic side
6. Neurotransmitters bind to receptor either:
i) ligand-gated ion channel or
ii) receptors linked to 2nd messenger systems
7. Binding results in a conductance change
- channels open or close or
- binding results in modulation of postsynaptic side
8. Postsynaptic response
- change in membrane potential (e.g. muscle contraction in the case of a
motorneuron at a neuromuscular junction)
9. Neurotransmitter is removed from the cleft by two mechanisms
i) transmitter is destroyed by an enzyme such as acetylcholine esterase
ii) transmitter is taken back up into the presynaptic cell and recycled
e.g. - acetylcholine esterase, breaks down acetylcholine in cleft, choline is
recycled back into the presynaptic terminal
Removal of neurotransmitter
a) broken down by enzymes
- acetylcholine esterase breaks down acetylcholine in the synaptic cleft
- many nerve gases and insecticides work by blocking acetylcholine esterase
- prolongs synaptic communication
b) recycled by uptake
- most neurotransmitters are removed by Na+/neurotransmitter symporters
- due to a specific neurotransmitter transporter
- recycled by uptake into presynaptic terminal or other cells
(glial cells will take up neurotransmitters)
c) diffusion, simple diffusion away from site
Presynaptic Vesicles
- neurotransmitters can be divided into two groups
i) low molecular weight, non-peptide
e.g. acetylcholine, glycine, glutamate
ii) neuropeptide (over 40 identified so far and counting)
- same transmitters found widely distributed through out diverse organisms
There are 2 types of secretory vesicles for this course we will only talk
about small chemical synaptic vesicles (the other class neuropeptides are made
and packaged in the cell body and transported to synapse).
Small chemical neurotransmitter vesicles
- responsible for fast synaptic signaling
- store non-peptide neurotransmitters,
e.g. acetylcholine, glycine, glutamate
- enough vesicles in the typical nerve terminal to transmit a few thousand
impulses
- exocytosis only occurs after an increase of internal Ca+2 (due to
depolarization) and at active zones (regions in the presynaptic membrane
adjacent to the cleft)
Figure 21-29, Lodish 4th edition. Figure 7-42, Lodish 5th edition. Release of neurotransmitters and the recycling of synaptic vesicles.
Vesicles import neurotransmitters (red circles) from the cytosol (step 1) using a H+/neurotransmitter antiporter. The low intravesicular pH, generated by a V-type ATPase in the vesicle membrane, powers neurotransmitter import. The vesicles then move to the active zone near the plasma membrane (step 2) and “dock” at defined membrane sites by interacting with specific proteins (step 3). A rise in cytosolic Ca2+ triggers fusion of the docked vesicles and release of neurotransmitters into the synaptic cleft (step 4). Synaptic-vesicle membrane proteins are then specifically recovered by endocytosis, usually in clathrin-coated vesicles (step 5). The clathrin coat is depolymerized, yielding vesicles that are the same size as synaptic vesicles. These new synaptic vesicles then are filled with neurotransmitters (step 1), completing the cycle, which typically takes about 60 seconds.
Neurotransmitter - goes
through a number of separate stages in its actions
1. Synthesis
- all small chemical neurotransmitters are made in the nerve terminal
- responsible for fast synaptic signalling
- synthetic enzymes + precursors transported into nerve terminal
- subject to feedback inhibition (from recycled neurotransmitters)
- can be stimulated to increase activity (via Ca+2 stimulated phosphorylation)
2. Packaging into vesicles
- neurotransmitters packaged into vesicles
- packaged in small "classical" vesicles
- involves a pump powered by a pH gradient between outside and inside of
vesicle
- pump blocked by drugs and these block neurotransmitter release
Vesicle Exocytosis
- many of the molecules that involved in vesicle exocytosis are now known
- related to other vesicle fusion systems such as Golgi or secretory
procedures
- a group of 6 to 7 proteins work together to respond to Ca+2 influx and
regulate vesicle fusion
- many of these proteins are common to other secretory pathways
- in yeast: mutations that affect the homologues of these exocytosis mediating
proteins have been made and all impair exocytosis
- after exocytosis the synaptic vesicle membranes are reinternalized by
endocytosis and reused (reloaded with neurotransmitter by a transmitter
transporter system)
- vesicles are also transported from the cell body to the nerve terminal
- transmitter is synthesized in the terminal and loaded into the vesicles
- enzymes and substrates necessary are present in the terminal
- i.e. acetylcholine, acetyl-CoA + choline used by choline acetyltransferase
i) non-peptide transmitters
- exocytosis only occurs after an increase of internal Ca+2 (due to
depolarization)
- at active zones (regions in the presynaptic membrane adjacent to the cleft)
- many of the molecules that involved in vesicle exocytosis are now know
- a group of 6 to 7 proteins work together to respond to Ca+2 influx and
regulate vesicle fusion
ii) peptide-transmitters (same as for non-peptide transmitters except:)
- exocytosis is NOT restricted to active zones
- exocytosis is triggered by trains of action potentials
SNARE hypothesis
Figure 21-31, Lodish 4th edition. Synaptic-vesicle and plasma-membrane proteins important for vesicle docking and fusion. Interaction of the T-SNAREs syntaxin and SNAP25 with the V-SNARE VAMP is aided by Rab3A. Neurexin, Ca2+ channels, and other plasma-membrane proteins localized to the synaptic region may also interact with synaptic-vesicle proteins. The vesicle protein synaptotagmin is the major Ca2+ sensor that triggers vesicle fusion
- four components
i) V-SNARE - vesicle membrane proteins
ii) T-SNARE - target membrane proteins
- vesicle docking occurs between the V-SNARE and T-SNARE proteins
- the combined proteins act as a receptor for an ATPase that utlizes ATP to
generate the "docked" form
- one of the proteins is a Ca+2 sensor such that when Ca+2 enters the synapse
the vescile fuses with the plasma membrane and releases its contents
- the membrane and proteins are then recycles through endocytosis (clatharin
coat and dynamin etc.) and reused.
Vesicle release can be blocked by toxins

From Purves, Neuroscience 3rd edition.
Each toxin cleaves a component of the SNARE complex and results in block of synaptic transmission. I.e. Ca+2 influx still occurs but vesicle release is reduced or blocked